Abstract
Background Treatment of relapsed and refractory multiple myeloma (RRMM) has been transformed by novel therapies including anti-CD38 monoclonal antibodies (mAbs) and 2nd and 3rd generation immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs), resulting in increasing numbers of triple-class exposed (TCE) patients (pts), defined as those who have received treatment with ≥1 IMiD, ≥1 PI, and ≥1 anti-CD38 mAb. Many such patients are exposed to ≥5 drugs in these classes (i.e., "penta-exposed", PE: ≥2 IMIDs, ≥2 PIs, and an anti-CD38 mAb). Some are refractory to ≥1 drug in each class ("triple-class refractory", TCR) and some are both PE and TCR (PE-TCR). Data on current real-world outcomes for the spectrum of heavily pre-treated, advanced RRMM patients are limited. The objective of this study was to examine patterns of treatment and healthcare resource utilization (HRU) and costs in commercially-insured patients with TCE RRMM in the US using a combination of two large health-insurance claims datasets: the IQVIA PharMetrics® Plus Health Insurance Claims database (data thru December 2020) and the Optum Clinformatics® Data Mart (data thru June 2021).
Methods Adult patients with continuous enrollment on or after December 1, 2015 (the date of daratumumab's approval by the US Food and Drug Administration) and a diagnosis of MM and evidence of being TCE during the continuous enrollment period were selected. TCE was defined as having received ≥1 IMiD, ≥1 PI, and ≥1 anti-CD38 mAb. For each patient, the 1st LOT after becoming TCE was identified ("index LOT") and the date of initiation of this LOT was defined as the "index date". Outcomes included: anti-MM treatments received for index LOT, time to discontinuation (TTD), HRU (numbers of outpatient visits, hospitalizations, hospital days), healthcare costs (adjusted to 2021 USD$, including costs of MM medications [including administration], costs of other MM-related care, non-MM-related care). TTD was analyzed by Kaplan-Meier (KM) methods. HRU and costs were reported on a per patient per month (PPPM) basis. Data from the 2 datasets were pooled directly (for PharMetrics, paid amounts were used as a proxy for costs). Analyses were descriptive.
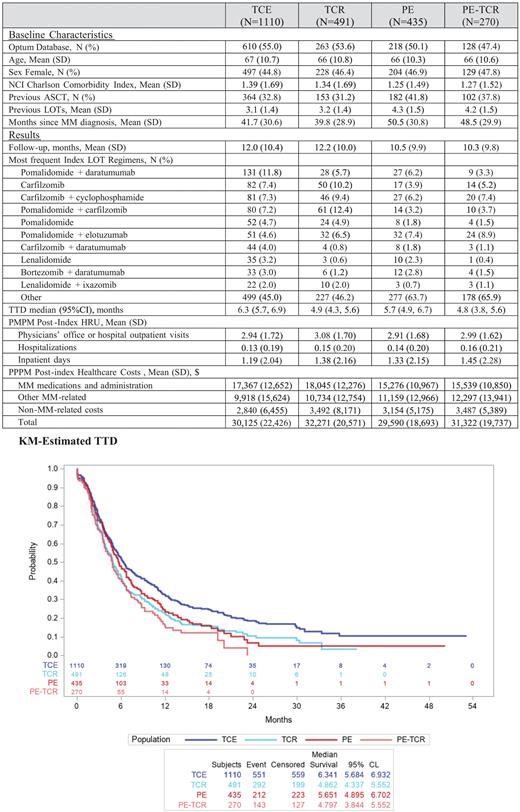
Results 72,933 patients with a confirmed diagnosis of MM from December 1, 2015 to the end of the study period were identified. Of these, 6,036 patients were age ≥18 years and were TCE; 1280 had continuous enrollment from 6 months prior to MM diagnosis through TCE date and had LOT after becoming TCE. After applying all other inclusion criteria, a total of 1,110 TCE, 491 TCR, 435 PE, and 270 PE-TCR patients were identified.
Across the four cohorts, mean age range was 66-67 years, 45-48% were female, 31% (TCR) to 42% (PE) had prior ASCT. The mean number of prior LOTs ranged from 3.1 (TCE) to 4.3 (PE). Despite decreasing numbers of MM patients over the study period, the numbers of new TCE patients increased almost 8-fold from 42 in 2016 to 319 in 2020; similar increases were observed for TCR, PE, and PE-TCR patients.
Mean (SD) post-index follow-up months was 12.0 (10.4) for TCE, 12.2 (10.0) for TCR, 11.0 (9.9) for PE, and 10.8 (9.8) for PE-TCR. There was no standard of care (SOC) treatment across the 4 cohorts; the most frequently utilized index LOT regimens were pomalidomide + daratumumab (12% TCE), pomalidomide + carfilzomib (12.4% TCR), and pomalidomide + elotuzumab (7% PE and 9% PE-TCR). The most frequently received medication during the index LOT for TCE and PE patients was pomalidomide (37.6% and 27.4%, respectively). For TCR and PE-TCR patients, the most frequently received medication was carfilzomib (40.5% and 27.8%, respectively). Median TTD ranged from 4.8 (PE-TCR) to 6.3 months (TCE). TCE patients had a PPPM average of 2.94 office/outpatient visits, 5.19 outpatient prescriptions, 0.13 hospitalizations, and 1.19 days in hospital. Mean PPPM total healthcare costs (2021 $US) were $30,125, including $16,686 (55%) for MM medications and $9,918 (33%) for other MM related care for TCE patients. Monthly HRU and costs for TCR, PE, and PE-TCR patients were similar to those for TCE.
Conclusions Despite increasing numbers of TCE, TCR, PE, and PE-TCR MM patients, there is no apparent SOC treatment regimen. With current treatments, TTD is short, HRU and costs are high, These data underscore the high unmet need for new therapies in this growing population.
Disclosures
Ma:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Song:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Delea:Alexion: Research Funding; Sanofi: Research Funding; Regeneron: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Otsuka: Research Funding; Oncimmune: Research Funding; Myovant Sciences: Research Funding; Moderna: Research Funding; InterMune: Research Funding; GRAIL: Research Funding; GlaxoSmithKline: Research Funding; Global Blood Therapeutics: Research Funding; Eidos: Research Funding; Amgen: Research Funding; Vertex: Research Funding; Tactile Health: Research Funding; Takeda: Research Funding; Seattle Genetics: Research Funding; MinervaX: Research Funding; Leo Pharmaceuticals: Research Funding; Karius: Research Funding; Ionis: Research Funding; Dynavax: Research Funding; Cerevel: Research Funding; ADC Therapeutics: Research Funding; Akcea: Research Funding; Akebia: Research Funding; AbbVie: Research Funding; Fishawack Health: Other: equity ownership; Policy Analysis Inc.: Current Employment, Other: equity ownership. Kroog:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Ge:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Chi:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Moynahan:Policy Analysis Inc., a wholly owned subsidiary of Fishawack Health: Current Employment. Weycker:Alexion: Research Funding; Abbvie: Research Funding; Policy Analysis Inc.: Current Employment, Other: equity ownership; Akebia: Research Funding; ADC Therapeutics: Research Funding; Pfizer: Research Funding; Otsuka: Research Funding; Oncimmune: Research Funding; Tactile Health: Research Funding; Seattle Genetics: Research Funding; MinervaX: Research Funding; Moderna: Research Funding; Leo Pharmaceuticals: Research Funding; Karius: Research Funding; Akcea: Research Funding; GRAIL: Research Funding; Vertex: Research Funding; Dynavax: Research Funding; Amgen: Research Funding; Cerevel: Research Funding; GlaxoSmithKline: Research Funding; Fishawack Health: Other: equity ownership; Takeda: Research Funding; Sanofi: Research Funding; Regeneron: Research Funding; Novartis: Research Funding; Myovant Sciences: Research Funding; Ionis: Research Funding; InterMune: Research Funding; Global Blood Therapeutics: Research Funding; Eidos: Research Funding. Rodriguez Lorenc:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal